Hypovolemia with peripheral edema: What is wrong?

Highlights
- increased capillary leakage normally accelerates lymphatic flow which normalizes the balance between the body fluid volumes, and might even increase the plasma albumin concentration (“interstitial washdown”) (View Highlight)
- The pathophysiology responsible for poor lymphatic return might involve a reduction of the interstitial hydrostatic pressure (Pi) or failure of the intrinsic contractility of lymphatic smooth muscle cells (View Highlight)
- Primary lymphatics coalesce into larger secondary lymphatic vessels and then into collecting lymphatics, the later which posses’ circumferential lymphatic smooth muscle cells that impart contractile forces and endothelial-lined valves that limit uni-direction fluid flow [15]. Lymphatic vessels are typically found near the arteriole-venous bundle. (View Highlight)
- Fluid in the interstitial space consists both of free fluid and fluid associated with the gel phase [16]. The fibers and bundles of collagen and other filamentous matrix proteins create a lattice that excludes most serum proteins creating the “excluded volume”, e.g., the total fluid volume where serum proteins cannot harbor. This limits serum proteins to a small volume of free fluid outside the gel phase [17, 18] and in some areas colloid osmotic pressure might be higher than expected. (View Highlight)
- Surface charges on macromolecules attract electrolytes that contribute to the net osmotic force exerted by proteins and proteoglycans, a process called the Gibbs-Donnan equilibrium [19]. The Donnan osmotic pressure contributes by approximately one third to one-half of the gel-related influence on Pif (View Highlight)
- most clinically relevant crystalloid infusion fluids differ only slightly in their electrolyte composition which have little, if any, effect on fluid kinetics [21] and, therefore, no demonstrable effects on interstitial fluid pressure. The role of extracellular proteoglycans on electrolyte buffering remains an unsettled hypothesis (View Highlight)
- General anesthesia creates a moderately severe maldistribution of fluid that is dominated by the strong inhibition of the diuretic response to volume expansion [6, 10]. The anesthesia-induced decrease of arterial pressure unloads baroreceptors which, in turn, activates renal sympathetic nerves [23]. These nerves increase sodium and water reabsorption and cause renal vasoconstriction, all leading to reduced urine output (View Highlight)
- Another finding during general anesthesia is that rate constant for fluid distribution to the slowly equilibrating interstitial pool is increased [10]. The precise mechanism is not known, but one possibility is that the low urine output maintains an expanded central (plasma) volume and therefore a higher capillary hydrostatic pressure (Pc) that forces fluid into the slow exchange pool (View Highlight)
- small amounts of crystalloid fluid (300 mL during 15 min) expand mainly the plasma volume [24] while larger infused volumes, combined with restricted urine flow, promotes the development of a bi-exponential plasma dilution curve, indicating the slowly developing and prominent extravascular distribution (View Highlight)
- A complementary hypothesis is that general anesthesia initiates a mild form of the interstitial molecular events that occur during inflammation. These events are detailed below but include sequestration of fluid in the gel phase, resulting from a reduction of the interstitial hydrostatic pressure (Pif), and inhibition of lymphatic pumping. A recent finding that indirectly supports this hypothesis is that poor return flow from the interstitium to the plasma remains for several hours after awakening from general anesthesia, while the sympathetic-induced inhibition of the diuretic response to volume expansion resolves almost instantly (View Highlight)
- Movement of free interstitial fluid into the lymphatic system is promoted by two types of tissue forces: extrinsic and intrinsic forces (View Highlight)
- Extrinsic forces result from tissue movement, for example, skeletal muscle contraction, breathing, peristalsis, arterial pulsations, and external massage. All these actions increase tissue pressure. The primary lymphatic vessels are capillary-like structures whose walls are made of loosely apposed endothelial cells that open in response to increased tissue pressure and allows fluid to enter the lymphatic capillaries. Compression garments aids in pushing fluid into the primary lymphatic vessels by increasing the tissue pressure. (View Highlight)
- Intrinsic forces refer to contractility of lymphatic smooth muscle cells. Lymphatic smooth muscle cells have characteristics of both smooth muscle and cardiac muscle cells which demonstrate spontaneous contractions and contractile forces that are modulated by preload, afterload, and contractility. In concert with uni-directional valves, intrinsic lymphatic pumping (rate and contractility) determines lymph flow rates. (View Highlight)
- There are clinical conditions in which volume kinetic studies show that the return flow of fluid from the interstitium to the plasma (probably via the lymphatics) is severely reduced. These are the "transurethral resection syndrome" (studied in pigs) [28], sepsis (sheep) [29], and preeclampsia (humans) [3]. These three conditions are all characterized by hypovolemia, hypoalbuminemia, and peripheral edema (View Highlight)
- Prior to the mid-1960’s, Pif was assumed to be near zero or slightly positive relative to the atmosphere. Guyton and colleagues tested a wide range of experimental conditions and consistently measured a negative Pif in the range of − 3 to 7 mm Hg [30, 31] which more recently has been reported to be − 2 to − 3 mm Hg (View Highlight)
- The negative Pif is maintained by an imbibition (suction) pressure created by colloids in the gel phase [20, 30, 31]. Moreover, the interstitial space is under tensile stress created by fibroblast-mediated, integrin-dependent processes (View Highlight)
- The mechanisms cited above keep the interstitial space dry and maintains a very low volume of free fluid, which has been suggested to occupy as little as 1% of the interstitial volume. The remaining interstitial fluid is bound in the gel phase (View Highlight)
- volume kinetic analyses suggest that the free fluid phase, which may also include the lymphatic volume, whole or in part, normally amounts to approximately the same volume as the plasma. However, it might occupy a smaller space in inflammatory conditions as more fluid becomes sequestrated in the slowly exchanging pool (View Highlight)
- Analysis of interstitial pressure and volume during lymphatic failure draws data primarily from studies of the skin, as it is among the largest organ system of the body and is estimated to hold approximately 3 L of fluid, second only to skeletal muscle that accounts for approximately 20 L (View Highlight)
- Peripheral edema is usually observed earliest in the skin and the interstitial space of the dermis and sub-dermis have been well characterized in terms of histology and physiology. (View Highlight)
- Injecting lipopolysaccharide or inflammatory molecules intravenously and, in some cases, directly into the skin, reduces Pif from −2 mmHg to between −7.5 and −10 mmHg within minutes [32, 34]. The most dramatic decrease in Pif occurs in burn injury, where values of 40 mmHg and greater have been reported [37,38,39,40,41]. Such pressure changes suck intravascular fluid into the interstitial space and further reduces lymphatic flow (View Highlight)
- The interstitial space is under tensile forces mediated by cell-collagen interactions and, specifically, by integrins. The basal tensile force sets the pressure–volume relationship. When tissue is excised and placed in isotonic saline it expands due to loss of these tensile forces (View Highlight)
- inflammatory mediators reduce Pif to more negative values. Numerous cytokines reverse integrin-collagen binding, including histamine (mast cell degranulation and Compound 48/80), PAF, TNFα, IL-1β, IL-6, LPS, and IFN-γ (View Highlight)
- 4
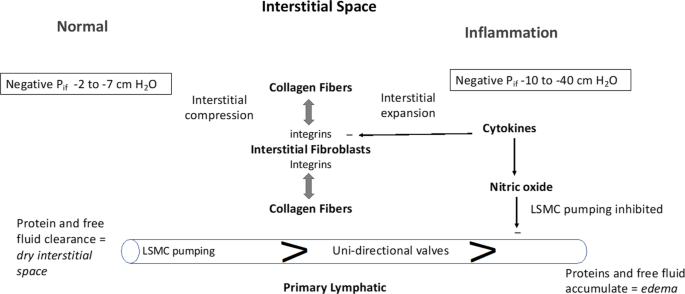
The interstitial space is represented in its Normal state (left side) and during Inflammation (right side). The interstitial space is normally contracted by integrin-dependent binding between interstitial fibroblasts and collagen fibers (tension indicated by double-headed arrows) that induces Interstitial Compression. In the Normal state, the interstitial space is kept “dry” by the action of lymphatic clearance and baseline pressure (Pif) is slightly negative in the range of −2 to −7 cm H2O. Lymphatic clearance is dependent on activity of lymphatic smooth muscle cell pumping and functional uni-directional valves. During Inflammation, cytokines induce fibroblast-dependent relaxation and disassembly of the integrin-collagen complex, that promotes Interstitial Expansion and a more negative pressure (-10 to −40 cm H2O). Nitric oxide produced during inflammation has direct inhibitory actions on lymphatic smooth muscle cells to reduce the rate and force of lymphatic smooth muscle contraction; the reduced lymphatic clearance of fluid and proteins results in edema (View Highlight)
- Fibroblasts bind to collagen via cell surface integrins that serve as protein linkages between the cell’s cytoskeleton and the collagen. The integrins are composed of heterodimers of α- and β-subunits, with both subunits having distinct members. There are four collagen-binding integrins: α1 β1, α2 β2, α10 β1, α11 β1 [43,44,45,46]. Fibroblasts generate tensile forces via integrin-collagen binding which contracts the interstitial space (View Highlight)
- During acute inflammation, there is a biphasic change in Pif. First, Pif becomes more negative due to the loss of tensile forces by cytokine-dependent interstitial relaxation. This expansion occurs rapidly and the ensuing more negative Pif increases fluid filtration and imbibes fluid from the capillary into the interstitial space. Pif starts at slightly negative pressures (− 2 to − 7 cm H2O) and might become deeply negative (− 10 to − 40 cm H2O). The shift can cause life-threatening hypovolemia during the first 6–8 h after burn injury [48] but negative changes in Pif are likely to be initially induced, to various degrees, in all conditions associated with acute inflammation. The presence of HA and glycosaminoglycans in plasma suggest that interstitial expansion is also associated with matrix fragmentation [49]. The second phase of this process—dominated by interstitial volume expansion shifts Pif towards zero, where the compliance characteristics for interstitial volume expansion cease (View Highlight)
- The interstitial edema and hypovolemia become aggravated if lymphatic pumping is impaired. During sepsis and states of sustained inflammation, increased levels of nitric oxide (NO), likely generated by inducible nitric oxide synthase (iNOS), inhibits lymphatic smooth muscle cell pumping and, thus, contributes to lymphatic failure [50,51,52]. Slow return of albumin-rich lymph is the likely cause of maldistribution of albumin during inflammatory conditions, as the synthetic rate of albumin is normal during major abdominal surgery [53] and sepsis [54]. Moreover, laboratory experiments show that anesthesia drugs inhibit lymphatic pumping (View Highlight)
- The combined effects of low Pif and poor lymphatic pumping greatly expands the interstitial space. As the interstitium fills with fluid, Pif shifts along its pressure–volume curve towards more positive values and eventually approaching zero, thus diminishing the forces for further fluid influx (View Highlight)
- During experimental thermal injury, the transition from largely negative Pif towards zero occurred over a time period of hours [40]. These changes allow fluid to enter the interstitial space with virtually no resistance, which causes pathological alterations in the interstitial architecture due to excessive amount of free fluid, e.g., ¨pitting¨ edema [35]. Lacune filled with fluid appears between the cells that may even lead to cellular hypoxia. The cytoskeleton of encapsulated organs might become disrupted, the severity of which varies slightly depending on the type of infused fluid (View Highlight)
- Reduction of Pif increases the capillary leakage of blood plasma by a suction effect that does not necessarily involve alteration of the capillary permeability. This was illustrated by Arturson and Mellander in 1964 who studied the effects of second-grade burn injury on the cat paw; they found accelerated loss of intravascular fluid to the injured area but no change in the capillary filtration coefficient, which is a measure of capillary permeability (View Highlight)
- A special case is the rare Idiopathic Capillary Leak Syndrome (Clarksons´disease) which presents dramatically with attacks of hypovolemic shock and pitting edema [58, 59]. Clarkson´s disease is not associated with inflammation [59] but acute serum from patients with Clarksons´s disease increases capillary leakage in experimental systems [60]. Polyvalent immunoglobulin is effective treatment, but endothelial hyperpermeability can also been counteracted by increasing intracellular cAMP using terbutaline (a beta2-receptor agonist) and theophylline (View Highlight)
- The kidney has an extensive network of specialized lymphatic vessels that are named for their intrarenal location [62]. Due to the renal capsule, Pif rises quickly as a result of external capsular compression, lymphatic obstruction (metastasis), systemic venous hypertension (e.g., congestive heart failure), ureteral obstruction, and increased capillary permeability. Parenchymal edema has been suggested to collapse the capsular and hilar lymph vessels and prevents lymph outflow, contributing to further pathological increases in Pif [63]. Renal interstitial edema and increased Pif, is a typical finding in acute kidney injury (AKI) and is commonly associated with elements of intra-renal lymphatic failure [64, 65] likely due to compressive obstruction. (View Highlight)

